|
|
The Product
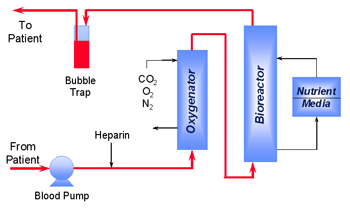
Our Bioartificial Liver System comprises an extracorporeal (outside the body) process for continuously withdrawing a patient's whole venous blood, maintaining temperature, oxygenating to arterial levels, adjusting pH to 7.2, and perfusing a hollow fiber bioreactor charged with primary porcine hepatocytes before returning the blood to the patient. Porcine hepatocytes are obtained from qualified animals produced in a high health status herd. Approximately 100 grams of hepatocytes are infused into the hollow fiber cartridge. Viability, oxygen consumption and other parameters are monitored to establish potency of each hepatocyte preparation and the resulting Bioreactor. The hollow fiber membrane, which serves as an immunoisolation barrier between the two species, is 1.7 m2 in surface area and has a nominal molecular weight cutoff of 100 kD. During clinical hemoperfusion, blood flow rates are maintained at 150 to 250 mL/min with a therapeutic procedure designed to last for 12 hours. The instrument console provides control of the process, detecting potentially hazardous conditions and alerting the operator to appropriate corrective actions. The bioreactor is disclosed in US Patent 5,955,353 issued in the U.S. in September, 1999. The patent describes a platform technology of high-density cell culture that can be extended beyond liver cells to a wide variety of other cell types including pancreatic islets and other endocrine cells. The Company's bioartificial liver system has also been designated an "Orphan Product" by the FDA for the treatment of acute liver failure. This offers additional proprietary protection for seven years after market approval. |
Click below for more info... 
|